Clinical Research
(CR-035) A Pilot Study of Lower Extremity Wounds Utilizing A Novel Nanoparticle Self-Assembling Peptide
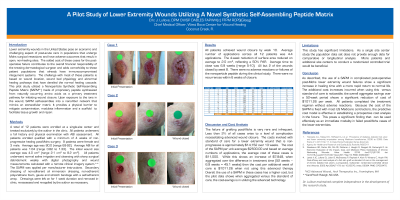
Methods:
The 10 (n=10) patients were seen from April 2023 to December 2023. All patients had failed post-Mohs primary closure and subsequently an additional 4-6 weeks of secondary healing prior to enrollment in the study. Use of the nanoparticle synthetic peptide was then applied at weekly intervals for 8 weeks to determine percent area closure. Secondary dressings were standardized for the pilot study and all patients underwent vascular testing before enrollment.
Results:
All 10 patients closed their wound by application #6. There was zero cases of recurrence and there were no adverse side effects or infections post-application. Average percent area reduction of wound size was reduced 67%. All wounds by week 8 were 100% closed.
Discussion:

.jpeg)