Case Series/Study
(CS-016) Enhancing Fasciotomy wound healing using Dehydrated Human Amnion/Chorion Membrane Allograft
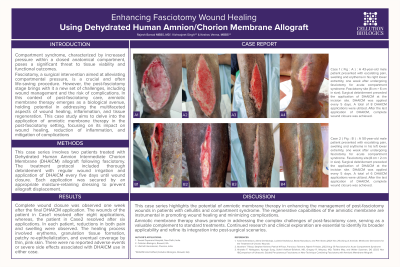
Compartment syndrome, characterized by increased pressure within a closed anatomical space, threatens tissue viability. Fasciotomy, while relieving this pressure, presents challenges in postoperative care, particularly in wound management and the risk of complications. In this context, amniotic membrane therapy, known for its healing, anti-inflammatory, and regenerative properties, emerges as a promising intervention. This case study examines the effectiveness of amniotic membrane therapy in post-fasciotomy patients, focusing on its role in enhancing wound healing and reducing complications. By analyzing specific cases, the study aims to provide valuable insights into post-fasciotomy care and highlight the potential of amniotic membrane therapy in improving outcomes for patients with compartment syndrome. Complete wound closure was observed one week after the final DHACM application. The patient in Case1 resolved post eight applications, while the patient in Case 2 resolved post six applications. In each patient, reductions in both pain and swelling were observed. The healing process involved erythema, granulation tissue formation, patchy re-epithelialization, and eventual coverage by thin, pink skin. There were no reported adverse events or severe side effects associated with DHACM use in either case. This case series highlights the potential of amniotic membrane therapy in enhancing the management of post-fasciotomy wounds in patients with cellulitis and compartment syndrome. The regenerative capabilities of the amniotic membrane are instrumental in promoting wound healing and minimizing complications. This therapy shows promise in addressing the complex challenges of post-fasciotomy care, serving as a valuable complement to standard treatments.
Methods: This case series involves two patients treated with Dehydrated Human Amnion Chorion Membrane (DHACM) allograft following fasciotomy. The treatment protocol included thorough debridement with regular wound irrigation and application of DHACM every five days until wound closure. Each application was secured by an appropriate moisture-retaining dressing to prevent allograft displacement.
Results:
Discussion:

.jpeg)