Case Series/Study
(CS-022) Healing complex neonatal dehisced surgical wounds with fish skin graft
Infants treated in the NICU are at increased risk of iatrogenic skin injury, including wounds of prematurity, congenital conditions, and dehisced surgical wounds.
Peristomal wound dehiscence is particularly challenging due to small and irregular space, peristomal dermatitis rendering skin very fragile, concern for colonization or infection and proximity of umbilical cord as well as monitoring devices. In addition, functionally and structurally immature neonatal skin heightens the concern for systemic absorption of certain topical antimicrobials and their safety in a neonate. Critical illness, suboptimal nutrient intake, and hypercatabolic state decrease calorie and protein available for wound healing. Frequent exposure to oxygen and lack of adequate oxygen radicals’ scavengers leaves tissues exposed to radical damage and inflammation.
Proving dehisced abdominal wounds with a stimulus to progress from inflammatory to proliferative state can be expedited with a matrix rich in anti-inflammatory messengers, pro-collagen stimulants, growth factors and anti-microbial messengers.
Methods:
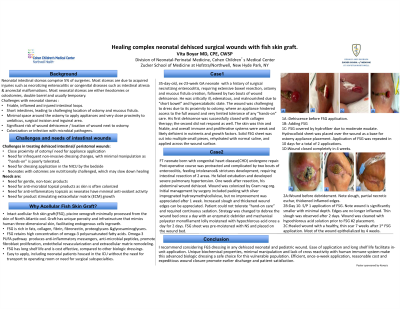
I report achieving closure of 2 dehisced peri-stomal wounds with such matrix: intact acellular fish skin allograft (FSG)*, processed from the skin of North Atlantic cod. Two neonatal cases of dehisced peristomal surgical wounds are described. Both had complex medical history and slowly healing trajectories. Wound 1 presented in a very preterm neonate with necrotizing enterocolitis, ostomy and 2 bouts of dehiscence. 2nd dehiscence was non-responsive to standard-of-care products. Wound 2 involved dehiscence after congenital imperforate anus repair and colostomy creation in a critically ill neonate with sepsis and organs failure.
Results:
Both required two FSG applications, 10 days apart, with simple secondary dressing coverages. Both wounds healed by 4 weeks with minimal manipulations.
Discussion:
This is a first report describing timely closure of dehisced abdominal wounds in critically ill, hypercatabolic and malnourished neonates. FSG is rich in fats, collagen, fibrin, proteoglycans, and glycosaminoglycans. Omega-3 polyunsaturated fatty acids and their metabolites are plentiful; they promote fibroblast proliferation, endothelial revascularization, and extracellular matrix remodeling. Anti-inflammatory messengers decrease propensity for hypertrophic scars. FSG unique porosity and infrastructure mimic human skin, facilitating cell ingrowth. Simple application allows in-unit care, minimizing stress of transport. Graft safety, ease of application and efficacious extracellular matrix growth make FSG excellent choice for complex neonatal wounds.

.jpeg)