Clinical Research
(CR-024) A multicenter, double blind, randomized, comparative clinical study to evaluate the efficacy and safety of diperoxochloric acid (DPOCl), a wound healing solution, in patients with chronic diabetic foot ulcer.
A clinical study was conducted to assess the efficacy and safety of diperoxochloric acid (DPOCl) in the treatment of diabetic foot ulcer.
Methods:
This study was designed as a multi-centre, double blind, randomised, parallel group comparison between 1.2 mM DPOCI and 0.9% saline solution. The study medications were administered locally on the wound dressing in a volume of approximately 0.2 ml per cm2 wound area per day for a maximum of 90 days.
Diagnosis and main criteria for inclusion:
Adult patients (aged 18 to 80 years) of both genders suffering from chronic diabetic foot ulcers (Wagner grade I and II Armstrong stadium A-C) of a diameter between 1.5 to 3.5 cm (corresponding to a maximal possible wound size at baseline between 1.8 and 9.6 cm2) after debridement which had been treated before unsuccessfully for at least 6 weeks. The study medication was to be administered up to complete wound closure or for a maximum of 90 days, whatever came first.
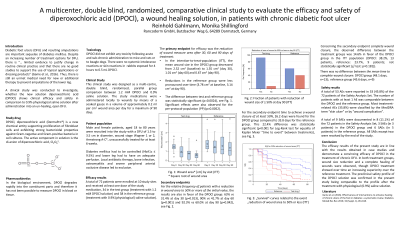
Results:
A total of 72 patients were enrolled at 10 study sites and received at least one dose of the study medication, 34 in the test group (treatment with 1.2 mM DPOCl solution) and 38 in the reference group (treatment with 0.9% physiological saline solution).
The primary endpoint for efficacy was the reduction of wound measure area after 30, 60 and 90 days of treatment. In the intention-to-treat-population, the mean wound size in the DPOCl group decreased from 2.52 cm2 (baseline) to 1.35 cm2 (day 30), 1.01 cm2 (day 60) and 0.67 cm2 (day 90). Reductions in the reference group were less pronounced over time (3.78 cm2 at baseline, 3.14 cm2 at day 90). The difference between test and reference group was statistically significant (p=0.0024). Significant effects were also observed for the per-protocol-population (p=0.0052). The numbers and types of the adverse effects in the DPOCl and reference group were similar.
Discussion:
Based on the results from this study, DPOCl is a promising new product for the treatment of diabetic foot ulcer.

.jpeg)