Clinical Research
(CR-017) The role of value analysis in pressure injury prevention: A Quality Improvement Project
Global pressure injury statistics reveal that hospital-acquired pressure injuries (HAPIs) remain a substantial burden, with over one in ten hospitalized adults being affected.1 PI are defined as an injury to the skin resulting from intense and/or prolonged pressure, or due to a combination of pressure and shear.2
The purpose of this analysis is to describe how the consistent collection, analysis, and use of data allows hospitals to validate their clinical and economic outcomes, and to adjust pressure injury prevention (PIP) strategies accordingly. This work recognizes the important role of Value Analysis Teams, which consider a variety of factors including clinical outcomes, product quality and comparisons, financial analysis, and education.3
Methods:
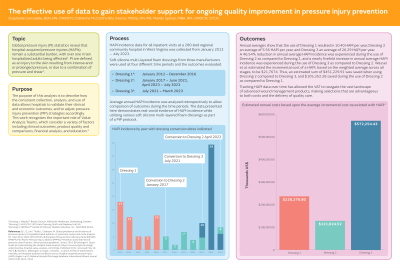
HAPI incidence data for acute care patients at a 280-bed regional community hospital in West Virginia was collected during the time period evaluated. Soft silicone multi-layered foam dressings from three manufacturers were used between the time period of January 2012 – July 2023 and the outcomes evaluated. Average annual HAPI incidence was calculated retrospectively to allow comparison of outcomes during the time periods that various soft silicone multi-layered foam manufacturers were used. The data presented here demonstrates real-world evidence of HAPI incidence while utilizing various soft silicone multi-layered foam dressings as part of a PIP protocol.
Results:
Annual averages show that the use of Dressing 1 resulted in 10.4 HAPI per year, Dressing 2 an average of 5.56 HAPI per year, and Dressing 3 an average of 26.29 HAPI per year. Additionally, an estimated sum of $451,233 was saved when using Dressing 2 compared to Dressing 3, and $105,449 saved during the use of Dressing 2 as compared to Dressing 1.
Discussion:
Monitoring data over time can validate product selection decisions to ensure they are advantageous to both costs and the delivery of quality care. Sharing of the data has led to hospital leadership and VAT support for investments in soft silicone multi-layered foam dressings as part of the broader PIP protocol.

.jpeg)